KESIMPTA® (ofatumumab) is the only self-administered B-cell treatment that offers patients a sense of control and independence when taking their medicine.1-3
.
Dosing
Give your patients the feeling of independence by taking their medication at home or on the go1*
.
*KESIMPTA Sensoready® Pens must be refrigerated at 2°C to 8°C (36°F to 46°F). Keep product in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming, do not shake. If necessary, KESIMPTA may be stored at room temperature below 30°C (86°F) for up to 7 days and returned to the refrigerator, to be used within the next 7 days.1
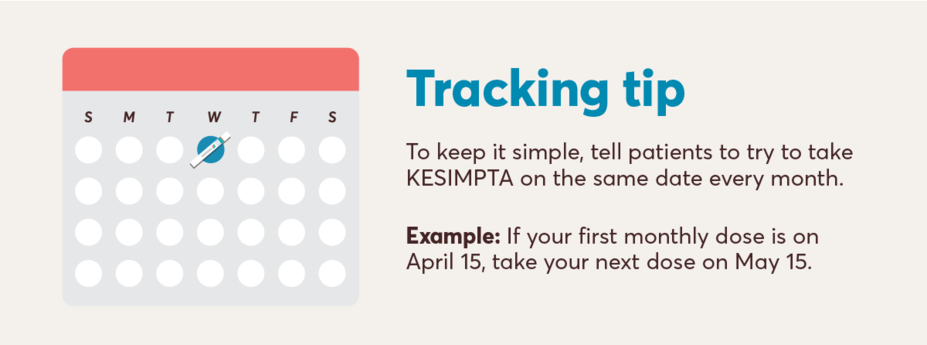
To help patients understand dosing with KESIMPTA, share the instructions below:
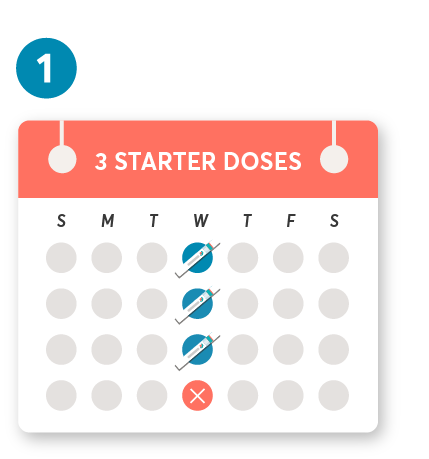
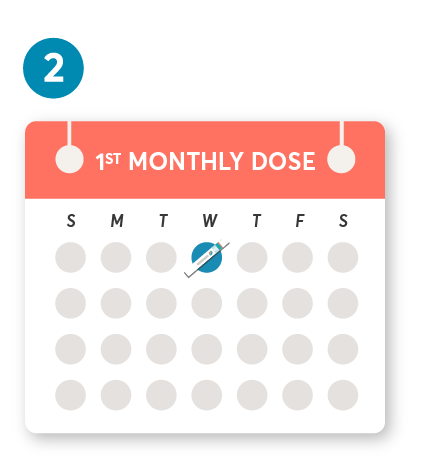
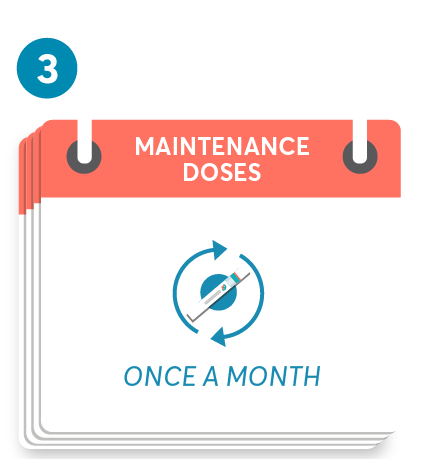
Initial dosing of 20 mg by SC injection at weeks 0, 1, and 2, followed by subsequent dosing of 20 mg by SC injection once monthly starting at week 4. After initial dosing, KESIMPTA is administered once monthly.1
First dose
The first injection should be performed under the guidance of a health care professional.1
Missed doses
Administer as soon as possible without waiting until the next scheduled dose; subsequent doses should be administered at the recommended intervals.1
The KESIMPTA Phase 2 trial determined that once monthly frequency in a low SC dose was comparably effective, with fewer side effects, vs higher SC doses.4,5
The KESIMPTA (20 mg) dose was chosen through dose modeling based on B-cell depletion results and Phase 2 data.4,5
See the Starting Patients page for information on screening and testing prior to starting treatment with KESIMPTA
The Pen
How KESIMPTA is administered
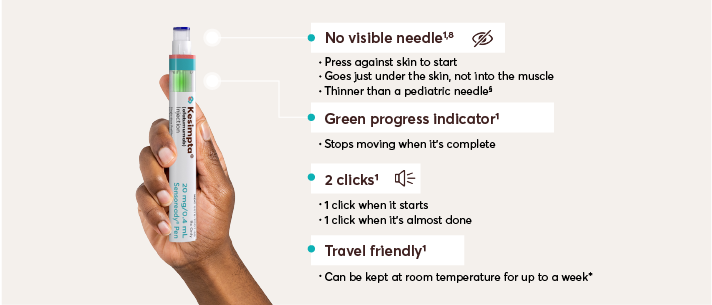
KESIMPTA is delivered via a pre-filled, easy-to-use autoinjector pen1,6,7†
Please see Instructions for Use for more detailed instructions on preparation and administration of KESIMPTA.
Show your patients how a real patient self-administers KESIMPTA
Watch Zenovia, a real patient, administer KESIMPTA with the easy-to-use† Sensoready Pen1,6
*KESIMPTA Sensoready Pens must be refrigerated at 2°C to 8°C (36°F to 46°F). Keep product in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming, do not shake. If necessary, KESIMPTA may be stored at room temperature below 30°C (86°F) for up to 7 days and returned to the refrigerator, to be used within the next 7 days.1
†Based on a cross-sectional survey of RMS patients (N=105) in the US who self-administered KESIMPTA with the Sensoready Pen within the previous 12 months. A total of 8 attributes of KESIMPTA Pen use were assessed, including overall ease of use, ease of device preparation, and portability. 89.5% of patients scored a 4 or 5 on characteristics of overall ease of use and ease of monthly dosing schedule. Questionnaire has not been validated. Initiation of KESIMPTA in patients may be influenced by insurance and availability (among other issues). Hence, data should be cautiously interpreted.6
‡Only limited benefit of premedication with corticosteriods, antihistamines, or acetaminophen was observed in RMS clinical studies. The first injection of KESIMPTA should be performed under the guidance of an appropriately trained health care professional. If injection-related reactions occur, symptomatic treatment is recommended.1
§Sensoready Pen comes with a staked needle of 29 gauge. Pediatric needles average between 23 gauge and 25 gauge.8,9
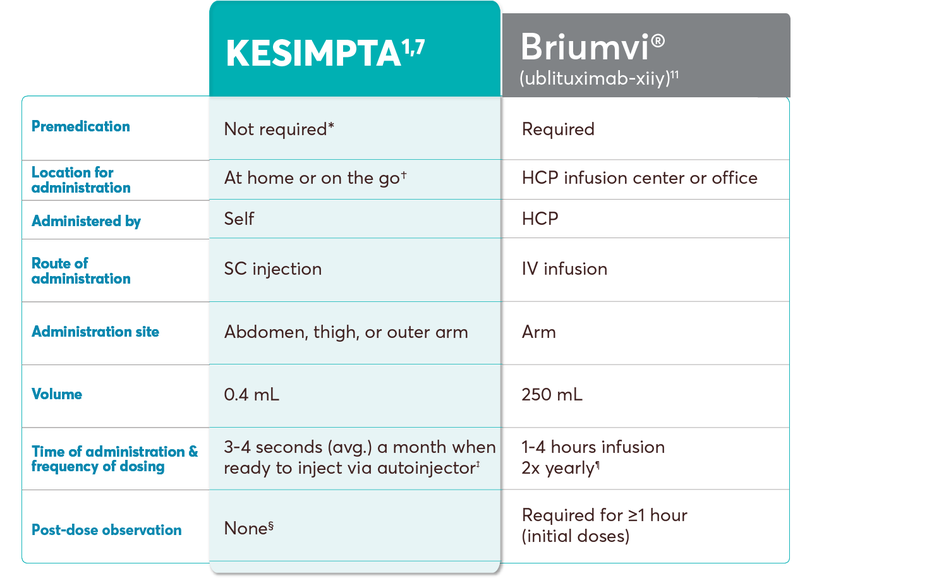
KESIMPTA dosing and administration vs other B-cell treatments
Click on image to enlarge
This chart is intended to show administration information only. Please refer to the product’s specific prescribing information for complete dosing and administration instructions.
No conclusions of comparative efficacy and safety should be drawn.
Click on image to enlarge
This chart is intended to show administration information only. Please refer to the product’s specific prescribing information for complete dosing and administration instructions.
No conclusions of comparative efficacy and safety should be drawn.
Click on image to enlarge
This chart is intended to show administration information only. Please refer to the product’s specific prescribing information for complete dosing and administration instructions.
No conclusions of comparative efficacy and safety should be drawn.
†KESIMPTA Sensoready Pens must be refrigerated at 2°C to 8°C (36°F to 46°F). Keep product in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming, do not shake. If necessary, KESIMPTA may be stored at room temperature below 30°C (86°F) for up to 7 days and returned to the refrigerator, to be used within the next 7 days.1
‡As per stability technical specification data, when the patient is ready to inject, it typically takes an average of 3-4 seconds a month to administer. Once-monthly dosing begins after the initial dosing period, which consists of 20-mg SC doses at weeks 0, 1, and 2. Please see Instructions for Use for more detailed instructions on preparation and administration of KESIMPTA.1,7
§The first injection of KESIMPTA should be performed under the guidance of an appropriately trained health care professional to monitor for injection-related reactions.1
‖The dose of Ocrevus of 600 mg every 6 months begins after the initial dosing period, which consists of two 300 mg IV infusions administered 2 weeks apart.10
¶The dose of Briumvi of 450 mg every 24 weeks begins after the initial dosing period, which consists of a first dose of 150 mg and a second dose of 450 mg administered as IV infusions 2 weeks later.11
See what patients are saying about the Sensoready Pen
Request KESIMPTA samples and a demo station to aid in your patient discussions
†Based on a cross-sectional survey of RMS patients (N=105) in the US who self-administered KESIMPTA with the Sensoready Pen within the previous 12 months. A total of 8 attributes of KESIMPTA Pen use were assessed, including overall ease of use, ease of device preparation, and portability. 89.5% of patients scored a 4 or 5 on characteristics of overall ease of use and ease of monthly dosing schedule. Questionnaire has not been validated. Initiation of KESIMPTA in patients may be influenced by insurance and availability (among other issues). Hence, data should be cautiously interpreted.6
Persistence Data
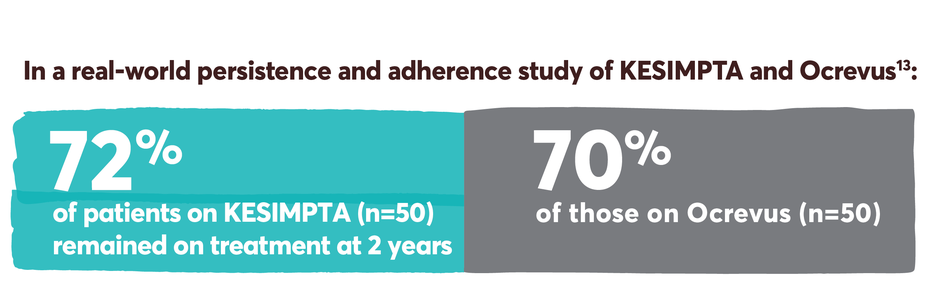
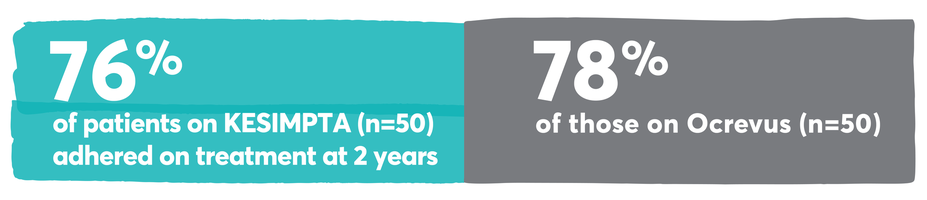
Patients who start KESIMPTA, stay on KESIMPTA
Persistence and adherence with KESIMPTA and other first-line options13-15
Persistence was defined as the time in months from the index date until treatment discontinuation. Discontinuation was defined as a gap ≥60 days in index therapy or switch to another DMT. The end date of supply prior to the gap was taken as the discontinuation date. Switch to another DMT was defined as ≥1 claim for another MS-related DMT, with the claim date of the other DMT taken as the switch date.13
Adherence was defined as the proportion of days covered (PDC) ≥0.8. PDC was calculated as the number of covered days in the period/the number of days in the period.13
Limitations:
1. Use caution when making any direct comparisons due to differences in DMT dosing schedules and pharmacodynamics
2. Claims data have inherent limitations:
1) There may be coding errors and missing data
2) Lack of data on reason for discontinuation
3) ICD-10 codes do not identify patients by MS subtypes. KESIMPTA is only indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults1
3. Early discontinuation may be overestimated if treatment occurred outside the purview of the claims data source13
KESIMPTA received approval in August 2020, resulting in a relatively limited number of patients with sufficient real-world follow-up data to assess persistence and adherence.13
Real-world persistence and adherence study design: Retrospective cohort study of adult RMS patients treated with KESIMPTA (n=59) or Ocrevus (n=205) conducted using Optum® Clinformatics® Data Mart Database (study period: August 2019–May 2023). Patients treated with KESIMPTA were matched 1:1 to patients treated with Ocrevus using greedy nearest neighbor propensity score matching without replacement and a caliper of 0.1. The propensity score was constructed from age at the index date (continuous), sex, race, region, number of RMS relapses in the preindex period (0, 1, 2+), prior DMT in the preindex period, RMS disability in the preindex period, Deyo-Charlson comorbidity index (DCCI) in the preindex period (continuous), and Psychiatric Diagnosis Group index in the preindex period (continuous).13
Inclusion criteria: The sample included adults (age ≥18 years) with13:
≥1 inpatient primary MS diagnosis or ≥2 outpatient MS diagnoses in any position ≥30 days apart in the 12 months prior to (preindex period) or 6 months following the index date
First claims were recorded on or after the index date of August 20, 2020, and continuous enrollment in a commercial insurance plan ≥12 months before and ≥24 months after the index date
The index date was defined as the date of the first KESIMPTA or Ocrevus claim, which was by design after the KESIMPTA US Food and Drug Administration approval on August 20, 2020
Optum® Clinformatics® Data Mart Database is a longitudinal database of medical and pharmacy administrative claims for patients enrolled in commercial insurance and Medicare Advantage plans in the United States.
For KESIMPTA, days of supply were determined directly from claims. Overlapping days of supply were accounted for by adding the extra days to the supply of the next dose. For Ocrevus, days of supply for each infusion was imputed as: min(182 days, [date of next infusion – date of current infusion + 1]).13
No conclusions can be drawn.
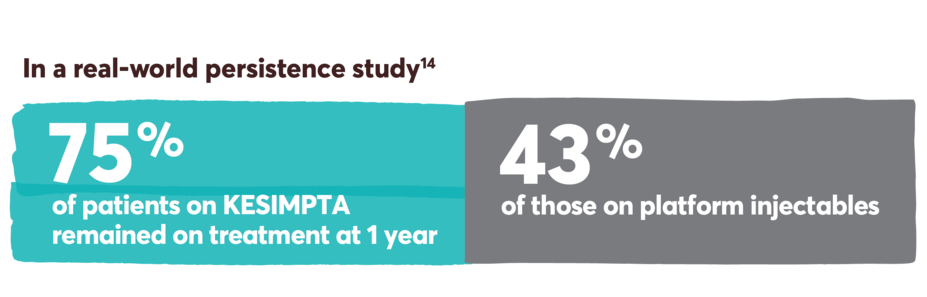
Persistence was defined as the number of days from the index date until discontinuation or a switch to a new DMT. It was measured during the post index period, which included the index date. Discontinuation was defined as a >60-day gap in therapy of the index medication, defined as a gap between the last supply date (based on expected duration of treatment or days’ supply) and the next claim date for the index therapy.14
Limitations: Analyses using claims data are dependent on the accuracy and specificity of information provided. Caution should be exercised in making any direct comparisons due to differences between DMTs. Findings may not be generalizable to other payor populations due to the study sample consisting primarily of commercially insured patients in the US. If treatment occurred outside of the purview of the claims data source, early discontinuation may be overestimated.
Retrospective cohort study of adult RMS patients treated with KESIMPTA (n=333) or platform injectables (n=333) (glatiramer acetate, interferon beta-1a/1b, and peginterferon beta-1a).14
This study was conducted from August 2020 to November 2021, utilizing the IQVIA PharMetrics® Plus database. Patients were indexed on first observed therapy and followed until discontinuation, switch, or 12 months post index for persistent patients. Propensity score matching was used to balance the baseline demographic, clinical, and RMS characteristics, as well as use of prior DMT between cohorts.14
IQVIA PharMetrics® Plus is a longitudinal health plan database of medical and pharmacy claims in the United States.
No conclusions can be drawn.
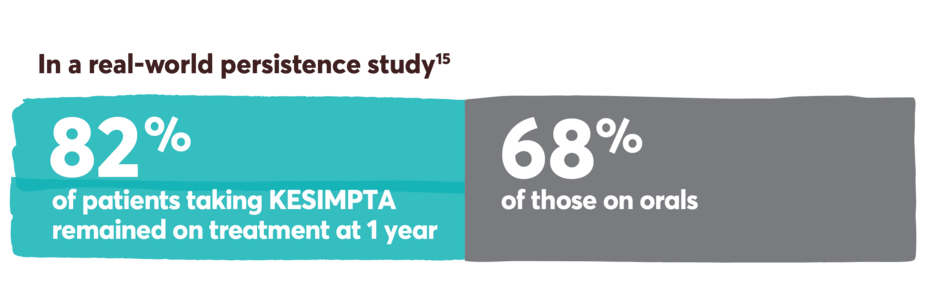
Persistence was defined as the number of days from the index date until discontinuation or a switch to a new DMT. It was measured during the post index period, which included the index date. Discontinuation was defined as a >90-day gap of the index medication, defined as a gap between the last supply date (based on expected duration of treatment or days’ supply) and the next claim date for the index therapy.15
Limitations: Analyses using claims data are dependent on the accuracy and specificity of information provided. Caution should be exercised in making any direct comparisons due to differences between DMTs. Findings may not be generalizable to other payor populations due to the study sample consisting primarily of commercially insured patients in the US. If treatment occurred outside of the purview of the claims data source, early discontinuation may be overestimated.
Retrospective cohort study of adult RMS patients treated with KESIMPTA (n=576) or oral DMTs (n=576) (dimethyl fumarate, fingolimod, teriflunomide, cladribine, siponimod, ozanimod, diroximel fumarate, monomethyl fumarate, and ponesimod).15
This study was conducted from August 2020 to November 2021, utilizing the IQVIA PharMetrics® Plus database. Patients were indexed on first observed therapy and followed until discontinuation, switch, or 12 months post index for persistent patients. Propensity score matching was used to balance the baseline demographic, clinical, and RMS characteristics, as well as use of prior DMT between cohorts.15
IQVIA PharMetrics® Plus is a longitudinal health plan database of medical and pharmacy claims in the United States.
No conclusions can be drawn.
Learn more about adherence and persistence from your peers

*Treatment-naïve was defined as patients not on any treatment prior to starting the clinical trials. This includes patients who started on KESIMPTA and patients who switched from Aubagio® (teriflunomide) to KESIMPTA.17